Amid the hype around green hydrogen, ammonia (NH3) is quietly emerging as another promising prospect for generating a storable energy carrier with the help of electricity from wind energy and photovoltaics. German industry has more than 100 years of experience in the production, storage and transport of this gas.

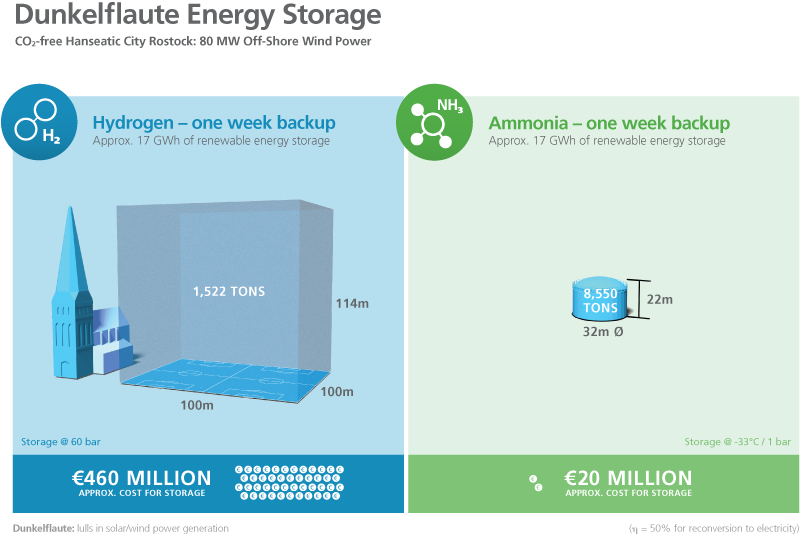
NH3 is an efficient medium for hydrogen storage. The hydrogen content of ammonia (108 g H2/l) is comparable to that of methanol (CH3OH). Unlike carbon-based energy carriers, ammonia can be produced without CO2, a gas that is present in the atmosphere at low concentrations. Compared to pure hydrogen, NH3 has the advantage of a significantly higher energy density per unit volume. Although H2 has the highest energy density per unit mass of all fuels (33.3 kWh/kg), ammonia has a significantly lower heating value, at 5.3 kWh/kg.
In practical applications, however, it is the volumetric energy density that is most important – and, for hydrogen, this value is extremely low (0.003 kWh/l) at atmospheric pressure. The energy density of liquid hydrogen is also very low (1.4 kWh/l), but this requires a pressure of approximately 700 bar. The volumetric energy density of liquid ammonia, on the other hand, is 3.2 kWh/l at a pressure of only 8 bar. This means that the amount of energy required to compress ammonia for liquefaction is much less than is required to compress hydrogen. However, since ammonia has a relatively low energy density compared to carbon-based liquid fuels (diesel: 9.9 kWh/l), it is mainly suitable as a fuel in shipping, rail or aviation vehicles, and for use in stationary energy supply systems.
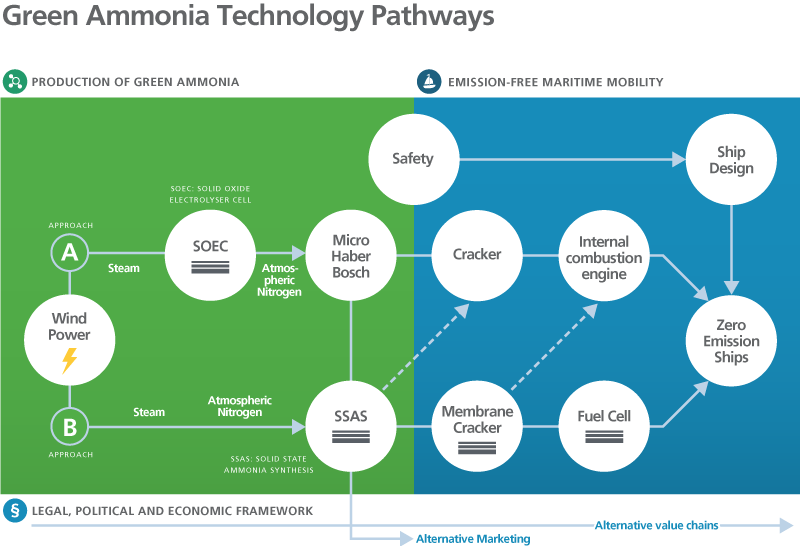
Ammonia is most commonly synthesised using the Haber-Bosch process, which involves several steps. First, molecular nitrogen must be obtained from the air, and the bonds between paired nitrogen atoms must be broken up in the high-pressure reactor (the ‘Haber-Bosch reactor’) using a catalyst under high-pressure and high-temperature conditions. Hydrogen is then produced from water vapor before undergoing a chemical reaction with nitrogen in the Haber-Bosch reactor to form ammonia. The energy required to produce hydrogen is mainly supplied by fossil fuels (for example, from natural gas via the process of steam reforming), which makes the process harmful to the climate and accounts for the roughly 3% of global CO2 emissions generated from fertiliser production. A ‘green’ alternative is to obtain hydrogen from the electrolysis of water vapor. If the energy required comes from renewable sources, the procedure is climate neutral. However, to be economically viable, current Haber-Bosch processes must operate continuously with a base load of at least 50%.
CAMPFIRE is developing load-flexible Haber-Bosch processes based on microstructured reaction technology, new catalysts, and innovative plant concepts so that, when fluctuating renewable energy is supplied, ammonia can be synthesised efficiently using hydrogen from electrolysis even at low base loads of 5–10%. Load-flexible Haber-Bosch processes can be used for on- and offshore storage of renewable energy. CAMPFIRE partners are also exploring a new possibility: a process that can produce NH3 directly from electrolysis. This means that the first step of hydrogen production can be skipped, and ammonia is produced directly from water and nitrogen in the air. In solid state ammonia synthesis (SSAS), water vapor reacts with ammonia at the cathode to produce ammonia. As a result, green ammonia can be produced particularly efficiently and in large quantities from wind or solar power.
Finally, in order to prepare the green ammonia for use as a fuel or convert it to molecular hydrogen, it is split back into hydrogen and nitrogen via an ‘ammonia cracker’. This conversion can take place either on shore or on board a ship. In the combustion engine, a mixture of ammonia and hydrogen provided from the cracker is used for an effective emission-free combustion process. A polymer-based fuel cell (polymer-exchange membrane fuel cell (PEMFC)) uses hydrogen produced in the cracker aboard the ship to generate energy for propulsion. The CAMPFIRE partners are developing highly dynamic ammonia crackers with an integrated step for fine hydrogen purification. Pure ammonia is converted into electricity in high-temperature solid oxide fuel cells (SOFCs). In the CAMPFIRE projects, the focus in this area is on the development of cost-effective cell concepts and synthesis processes that would lower the cost of SOFCs for high-output classes.
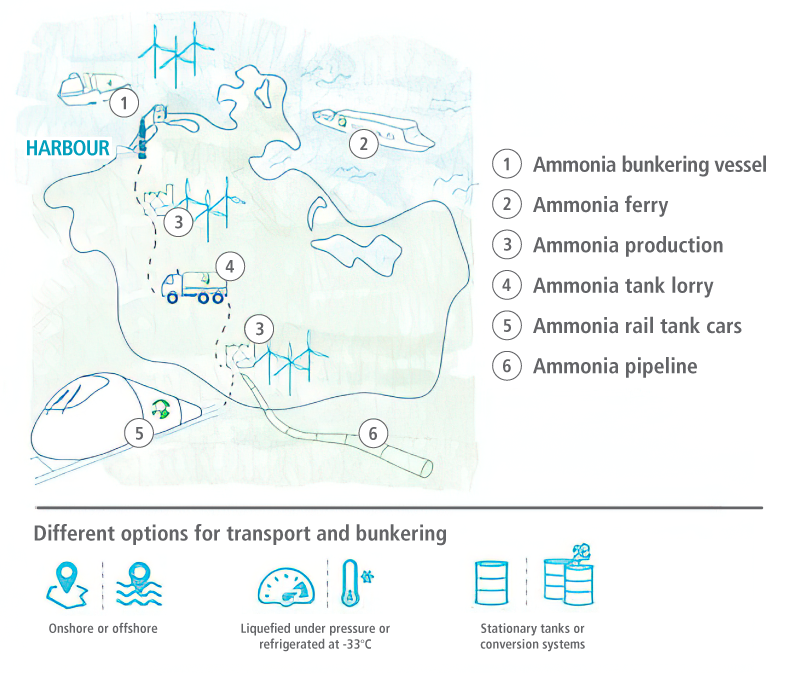
For the development of future transport chains based on green ammonia, CAMPFIRE is developing solutions for flexible, autonomous and emission-free refuelling systems – for ship-to-ship, land-to-ship and lorry-to-ship. The refuelling process must be feasible at different pressure and temperature levels. For hydrogen end users, ammonia-powered multimodal refuelling stations with adapted ammonia crackers and hydrogen fine cleaning modules are being developed to supply land-based consumers (cars, trucks, lorries, trains). For stationary energy supply, ammonia can be used directly in motorised combined heat and power (CHP) systems. For this purpose, CAMPFIRE is adapting the combustion process in CHP gas engines for hybrid operation with the cracker.