Ammonia is best known for its role in industrial fertiliser. It may also be the key to a low-carbon energy system.

Ammonia is more than just a building block for industrial fertiliser – it’s also an energy source. This isn’t science fiction! There were ammonia-powered streetcars in New Orleans as early as the 19th century and buses that ran on ammonia in Belgium in the 1940s. The gas could even be used to fuel the 1981 Chevrolet Impala, a passenger car manufactured in the US. In the future, ammonia will primarily be used in ships. It’s extremely important to develop alternative fuels for shipping, because the fuel that has so far been the most widely used, heavy fuel oil, is also the fuel with the worst effects on the climate. The combustion of ammonia, on the other hand, doesn’t produce any air pollutants; it only produces nitrogen and water vapor.
Ammonia is one of the most widely produced basic chemicals in the world. Its chemical formula is NH3, which means that it consists of one nitrogen and three hydrogen atoms. In the most commonly used production process, the Haber-Bosch process, the gases nitrogen and hydrogen react under high pressure (150–300 bar) and high temperatures (400–500 °C) on an iron catalyst. The chemical equation for the reaction is N2 + 3H2 → 2NH3. But this process, which was developed at the beginning of the 20th century, is harmful to the climate: the reforming reaction used to produce hydrogen from coal or gas releases greenhouse gases.
Hydrogen can be produced in a climate-friendly way through electrolysis, with the electricity needed for the process generated from renewable energies. This process first splits water into oxygen and hydrogen. A catalyst is then used to split nitrogen molecules into nitrogen atoms, which react with the hydrogen to form NH3. Although this process is climate-friendly, the production costs are relatively high. The CAMPFIRE project is testing a new method to produce green ammonia from wind and solar power. This process uses ceramic thin-film membranes to synthesise NH3 directly, which increases efficiency and improves cost-effectiveness.
Climate-friendly method
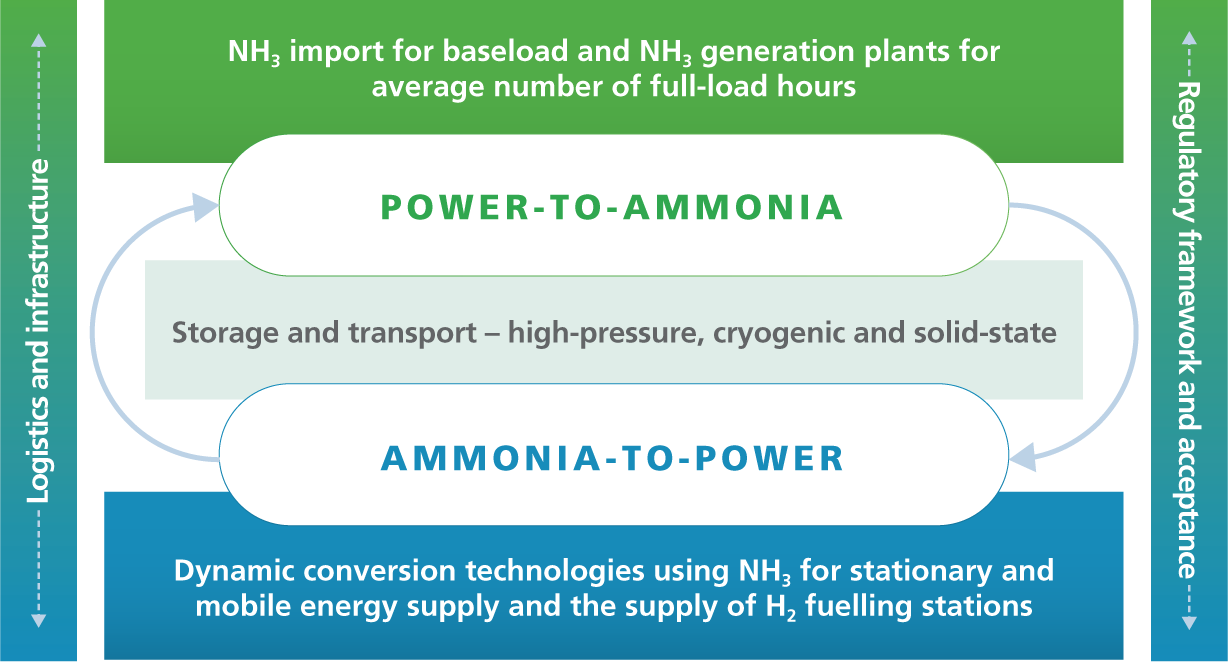
Compared to carbon-based synthetic energy sources like methane or methanol, nitrogen-based green ammonia has a key advantage: nitrogen is the main component of the atmosphere, accounting for just under 80%, while carbon is present in the air only in the form of carbon dioxide, which accounts for as little as 0.4%. Compared to pure hydrogen that has already been liquefied, liquid ammonia offers the advantage of a higher energy density: it contains 50% more energy per cubic metre of volume. Because ammonia liquefies at 33°C rather that at 253°C, it is also easier to store and transport. The consortium is developing important research interfaces across the board for a future ammonia ecosystem that encompasses the complete generation, logistics and reconversion chain. Innovative CAMPFIRE power-to-ammonia and ammonia-to-power technologies, refuelling technologies, and logistics and infrastructure concepts can overcome some of the most significant remaining obstacles to the green hydrogen economy of the future.