Thin films CF04_4
Project costs: 750 T€
Project Period: 07/2022–06/2024
Partners:
![]()




Overview:
Water electrolysis is a key technology for splitting water into hydrogen and oxygen using renewable energies. Green hydrogen is considered a promising energy carrier for a sustainable future and is produced by electrolysis using renewable energies.
In the CF04_4 project, the innovative approach of using anion exchange membranes for water electrolysis (AEM-WE) is being pursued, whereby the advantages of proton exchange membrane electrolyzers (PEM-WE) and alkaline electrolyzers (AEL) can be combined. On the one hand, expensive precious metals are no longer required to prevent corrosion and, on the other, no concentrated electrolyte is needed as the ion exchange takes place via a polymer-based anion exchange membrane.
The heart of an AEM-WE is the electrolysis cell based on a membrane electrode assembly (MEA). High-entropy alloys (HEA) are suitable as a new, precious metal-free material class for the catalysts. HEAs are alloys that consist of at least five homogeneously distributed metallic elements. This results in new properties that can be used in particular for electrocatalytic activity. This high activity in the hydrogen evolution reaction under alkaline conditions provides the precious metal-free catalysts with new, highly scalable manufacturing processes that can be used in industry.
Using atmospheric pressure plasma spraying, the porous nickel-based Raney cathode is developed. The cathode is then decorated with HEA particles, which are deposited from the homogeneously spark plasma sintered targets by magnetron sputtering. The overall aim of the CF04_4 project is to develop a functional model of a low-cost, high-performance, precious metal-free MEA for alkaline water electrolysis.
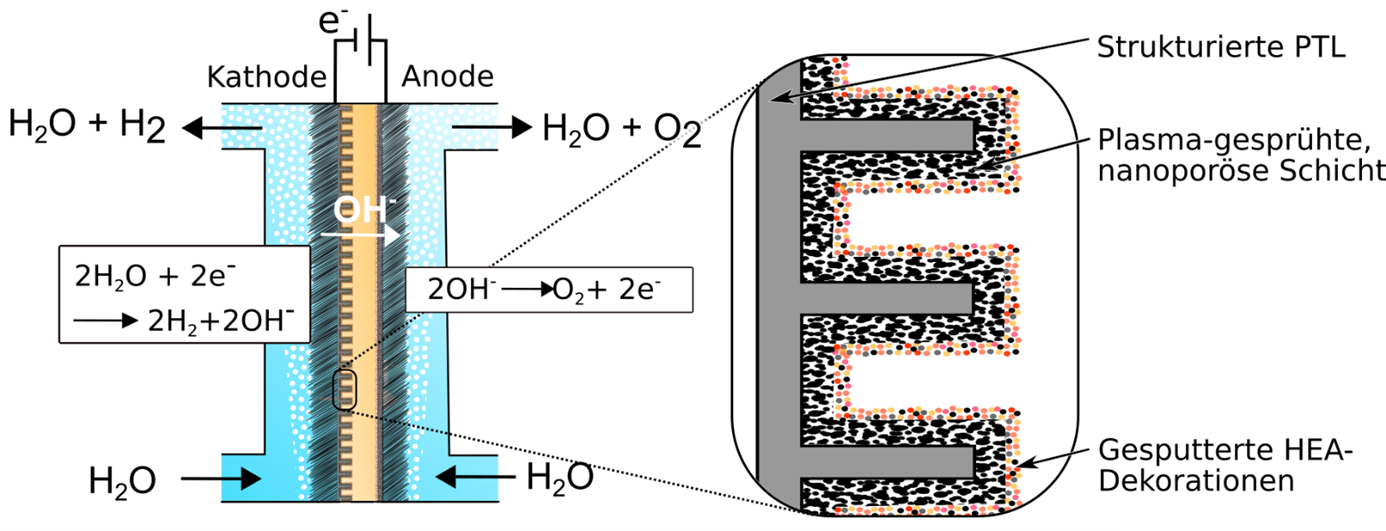
Design and functional principle of a membrane electrode unit with structured, porous transport layers (PTL) on the cathode side.










